Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Can We Use N-Acetylcysteine as An Antioxidant In SARS-Cov-2 Treatment? A Pathophysiology Review
*Corresponding author: Rawan Eskandarani, Emergency Medicine Consultant, King Fahad Medical City, Riyadh, Saudi Arabia.
Received:March 03, 2022; Published: March 31, 2022
DOI: 10.34297/AJBSR.2022.16.002183
Introduction
The Coronavirus disease of 2019 (COVID-19) was first identified in Wuhan (Hubei, China) in December 2019 [1] the virus responsible for this disease was identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In March of 2020, it was declared by the World Health Organization as a pandemic [2]. Within the coronavirus (Coronaviridae) family, the Alphacoronavirus and Beta coronavirus subfamily are known to be transmissible to humans [3]. Clinical symptoms vary widely, but the Beta coronavirus strains MERS-CoV (Middle East respiratory syndrome coronavirus), SARS coronavirus (severe acute respiratory syndrome coronavirus) now named SARS-CoV-1, and SARS-CoV-2 (the causing agent for COVID-19) seems to be of the most pathogenic strains of the group [4].
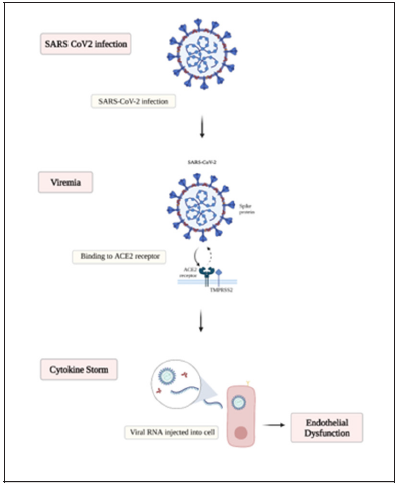
The clinical picture and presentation are widely ranged and can vary from asymptomatic carrier to the drastic extreme acute respiratory distress syndrome and death. in between the clinical presentation of fever, fatigue, lethargy, myalgia, dyspnea, headache, and diarrhea are frequently found symptoms among those patients who test positive for SARS-CoV-2 [5]. Multiple risk factors have been suggested as an increase in the risk of morbidity and mortality in a population such as advanced age, obesity, and prior medical illness [6-9]. In one case series in China, the mortality was 86% among those critically ill who required mechanical ventilation, and 79% among those who only required noninvasive ventilation [8]. The underlying pathophysiology of Covid-19 infection critical outcomes is still to be understood. It has been demonstrated that Covid-19 binds to the cell membrane protein angiotensin-converting enzyme 2 (ACE2) via its spike (S) glycoprotein with higher affinity than the severe acute respiratory syndrome (SARS)-CoV-1 [10] after binding to the cells Sars Covid-19 undergoes protein priming by cellular surface proteases, such as transmembrane protease serine 2 (TMPRSS2) allowing the entry and replication of the Covid-19 virus in the target cell [11] (Figure 1).
Many Covid-19 positive patients have been noted to have a significant increase in serum ferritin, C-reactive protein, erythrocyte sedimentation rate, and lactate dehydrogenase [12]. This is thought to be related to increased inflammation and stress in the body. In one computational study, they theorized that the increased ferritin marker in those patients is a protective mechanism related to the increased release of iron from the dissociation of heme into iron and porphyrin [13]. The heme group is a very large group of metalloproteins that is very important in different biological roles such as carrying oxygen, electron transfer, and other important biological processes. It is commonly present in hemoglobin, but also present in many other hemeproteins such as myoglobin, cytochromes, and catalases. In this study, they hypothesized that the Covid-19 virus attacks the hemoglobin and binds to porphyrin causing an increase in stress markers and disrupting the normal oxygen-carrying capacity and increasing the oxidative stress [13]. This theory is only based on computational methods and has been criticized and still lacks any further experimental methods to support it [14].
The cytokine storm that is described commonly with Covid-19 infection is related to the powerful activation of the immune system. The immune response and the release of pro-inflammatory cytokines IL-1alpha and IL-1beta, IL-6, IL-18, interferons (IFNs), Tumor necrosis factor (TNF), and chemokines (CXC, CC, C, and CX3C) are likely to be related to the pulmonary inflammation and extensive lung involvement in Covid-19 infection, leading to the acute respiratory distress syndrome (ARDS), coagulopathy and death [15-17]. The inflammatory response that activates the signaling pathway coordinates and regulates the pro- and anti-inflammatory mediators in tissue cells [18]. Proinflammatory cytokines such as TNFα and IL-1 when activated lead to triggering the nuclear factorkappa (BNF-κ) signaling pathway [19]. The activation of the NF-κB pathway has an important role in the pathogenesis of inflammatory diseases [20, 21]. when stimulated by the release of TNFα, or other cell stressors, the BNF-κ pathway can have a neuroprotective or a proinflammatory role depending on influence cell regulation and survival based on the tissue location and pathological state [18].
Other studies have demonstrated inhibition of NF-κB in macrophages resulted in the release of cytochrome c in the cytoplasm, which is an important key step in triggering cell apoptosis [22]. The binding of the Covid -19 virus to the ACE 2 receptors leads to the accumulation of angiotensin II which can also activate the (BNF-κ) and IL-6 pathway [23]. Angiotensin II accumulation will lead to increased vasoconstriction, increased oxidative stress, inflammation, and fibrosis [24]. The ACE2 receptors are concentrated in type II alveolar cells, macrophages, bronchial, and tracheal epithelial cells [25] (Figure 2). Normally, angiotensin II is converted to angiotensin 1,7 by the ACE2 receptor. when they are inhibited by the viral binding this results in decreased angiotensin 1,7 and its regulatory mechanism, causing a disruption in the balance of the major components of the renin-angiotensinaldosterone system (RAAS) [24].
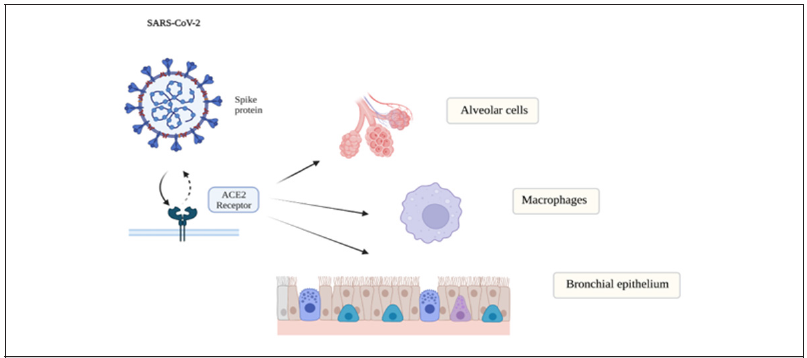
Figure 2:The ACE2 receptors are concentrated in type II alveolar cells, macrophages, bronchial and tracheal epithelial cells.
This inflammatory damage is further exacerbated by the oxidative stress caused by increased reactive oxygen species (ROS). Animal studies have demonstrated the enhanced ROS expression with disturbance in the function of the antioxidant systems which along with different factors can tremendously affect and exacerbate the host cell response [26]. Oxidative stress, mainly Toll-like receptor 4 (TLR4) pathway, is thought to be triggered by viral pathogens such as Covid-19 virus amplifying host inflammatory response and leading to Acute Lung Injury (ALI) [27]. In another animal study, they have linked acute lung injury to the local generation of reactive oxygen species and the subsequent formation of oxidative phospholipids. In this study, it was noted that loss of IL-6 will result in alleviating acute lung injury [26, 27].
The Covid-19 triggers the ROS which plays a key role in the initiation phase of Acute Respiratory Distress Syndrome (ARDS). The disruption in ACE2/ nitric oxide pathway will result in the inability of blood vessels to dilate, and aldosterone stimulation that will further exacerbate edema formation locally in the lung. This will cause an increase in pulmonary arterial pressures and worsen gas exchange [28]. NADPH oxidase mediates ROS production, and aldosterone induces NADPH oxidase of macrophages via the mineralocorticoid receptor (MCR) activation [26]. The lung inflammation, alveolar damage, and increased endothelial permeabilities all are noted when developing (ARDS) [29]. Neutrophils are also recruited by the released pro-inflammatory cytokines TNF-α, interleukin 1 beta (IL-1β), interleukin 6 (IL- 6), and interleukin 8 (IL-8), which in turn becomes activated and release further toxic mediators [30]. NF-κB plays a key role in mediating the inflammatory response and regulation of the antiinflammatory gene expression [31]. It is a multi-protein complex that has a main role in activating and triggering multiple cellular defense mechanisms [32].
The extensive free radical mediators and ROS can easily overwhelm the antioxidant system resulting in disseminated oxidative cell damage in the lung tissues. The increased production of ROS is associated with increased alveolar damage and histamine release from mast cells and increased mucus secretion from damaged airway epithelial cells [33] (Figure 3). In Acute lung injury, the oxidation of a variety of crucial proteins in the alveolar space has been shown to disrupt alveolar function and increase inflammatory stimuli, causing further damage. The high fraction of inspired oxygen (FiO2) is associated with increased oxidative stress and increased production of NO and O2• [32]. Using antioxidant therapies that can restore depleted mechanisms such as glutathione (GSH), N-acetyl-cysteine (NAC) have been all reported to be used to replenish antioxidants mechanisms in the body and regulate NF-κB signaling [34, 35].

Figure 3 : NF-kB, IL-6, TNF-α mediating the inflammatory response. ROS production contributes to alveolar damage and mast cell histamine release.
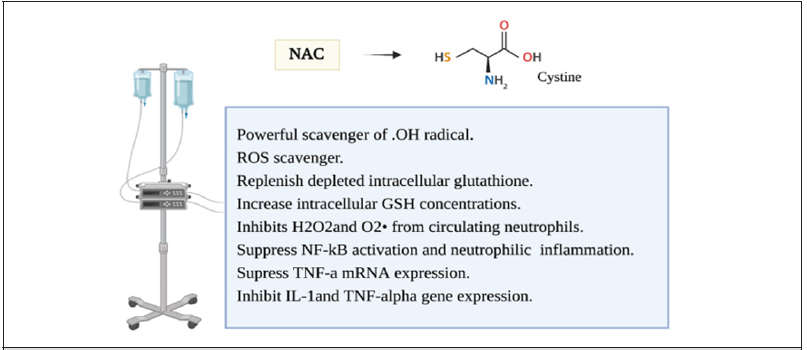
During the metabolism of molecular oxygen several free radicals are made en route before its complete reduction to H2O. This involves hydrogen peroxide (H2O2), hydroperoxyl radicals (OH-), and Superoxide radical (O2-). To be able to provide maximum protection, most of the cells have multiple mechanisms for scavenging different free radical species within the subcellular organelles [36]. Various intracellular cytosolic defense mechanisms have been identified and are crucial in maintaining cellular integrity and suppression of free radical reactions. The most important biological intracellular antioxidants are glutathione peroxidase, Vitamin E, Vitamin C, catalase, and superoxide dismutase [37]. In normal circumstances, the body can counteract oxidative stress. other than enzymatic antioxidants, a thiol reducing buffer (sulfhydryl containing compounds) consists of small proteins with redox-active sulfhydryl moieties such as glutathione and thioredoxin [38]. The glutathione is low molecular weight Thiol, Glutathione is synthesized from glutamate, cysteine, and glycine constituting a tripeptide that is considered the most abundant sulfhydryl-containing compound [39]. Most of the cellular GSH is present in the cytoplasm with the remainder in different organelles. N-acetylcysteine (NAC) is a thiol, a mucolytic, a precursor of L-cysteine, and reduced glutathione [38]. It is a powerful scavenger of OH radical. It has multiple mechanisms that have been studied as a ROS scavenger or an anti-inflammatory prooxidant agent [38, 40] (Figure 4). NAC have been demonstrated to replenish depleted intracellular glutathione in overwhelmed cellular oxidative stress situations [41]. It acts to increase intracellular GSH concentrations in alveolar macrophages and as a cysteine donor which is the rate-limiting step in GSH synthesis. It inhibits H2O
sub>2 and O2 from circulating neutrophils [32].It has also been shown to suppress NF-kB activation and neutrophilic lung inflammation and TNF-a mRNA expression which attenuated endotoxin-induced neutrophilic alveolitis [42, 43]. (NAC) was also shown to inhibit IL-1and TNF-alpha gene expression in inflammatory arthritis where ROS production was demonstrated to be inhibited by (NAC) and stimulated by (H2O2) which constitute second messengers for IL-1 and TNF-induced JNK activity [38, 40, 44, 45]. The use of NAC in Acute respiratory distress syndrome (ARDS) is not a new concept. This has been an attractive strategy in the treatment of (ARDS) but the benefit is still to be proved [46]. Despite the growing evidence of oxidant stress impact on hypoxia and inflammatory genes, the use of antioxidant treatment in critically ill patients is yet to be explored. Studies mostly failed to demonstrate the best way to utilize these therapy options, and which subgroup population would benefit the most from it. This is probably related to diminishing the immune response in these patients where it is most needed to overcome the illness [46, 47].
Acknowledgment
None
Conflict of Interest
None
References
- Valencia Damian N (2020) Brief Review on COVID-19: The 2020 Pandemic Caused by SARS-CoV-2. Cureus 12(3): e7386.
- Coronavirus n.d. (2020) Accessed June 5.
- Woo Patrick CY, Ming Wang, Susanna KP Lau, Huifang Xu, Rosana WS Poon, et al. (2007) Comparative Analysis of Twelve Genomes of Three Novel Group 2c and Group 2d Coronaviruses Reveals Unique Group and Subgroup Features. J Virol 81(4): 1574-1585.
- WHO (2015) Severe Acute Respiratory Syndrome (SARS), July.
- Wang Dawei, Bo Hu, Chang Hu, Fangfang Zhu, Xing Liu, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 323(11): 1061-1069.
- CDC COVID-19 Response Team (2020) Severe Outcomes among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 69(12): 343-346.
- Zhou Fei, Ting Yu, Ronghui Du, Guohui Fan, Ying Liu, et al (2020) Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 395(10229): 1054-1062.
- Huang Chaolin, Yeming Wang, Xingwang Li, Lili Ren, Jianping Zhao, et al. (2020) Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Onder Graziano, Giovanni Rezza, and Silvio Brusaferro (2020) Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 323(18): 1775-1776.
- Wrapp Daniel, Nianshuang Wang, Kizzmekia S Corbett, Jory A Goldsmith, Ching Lin Hsieh, et al. (2020) Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 367 (6483): 1260-1263.
- Hoffmann Markus, Hannah Kleine Weber, Simon Schroeder, Nadine Krüger, Tanja Herrler, et al. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor Cell 181(2): 271-80.e8.
- Chen Nanshan, Min Zhou, Xuan Dong, Jieming Qu, Fengyun Gong, et al. (2020) Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 395(10223): 507-513.
- Wenzhong Liu, Li Hualan (2020) COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. ChemRxiv.
- Read Randy (2020) Flawed Methods in COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. ChemRxiv.
- Tisoncik Jennifer R, Marcus J Korth, Cameron P Simmons, Jeremy Farrar, Thomas R Martin, et al. (2012) Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev 76 (1): 16-32.
- Channappanavar Rudragouda, Stanley Perlman (2017) Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin Immunopathol 39(5): 529-539.
- Coperchini Francesca, Luca Chiovato, Laura Croce, Flavia Magri, Mario Rotondi, et al. (2020) The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/chemokine-Receptor System. Cytokine & Growth Factor Reviews 53: 25-32.
- Albensi Benedict C (2019) What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Frontiers in Cell and Developmental Biology 7: 154.
- Lawrence Toby (2009) The Nuclear Factor NF-kappaB Pathway in Inflammation. Cold Spring Harb Perspecti Biol 1(6): a001651.
- Holgate Stephen T (2004) Cytokine and Anti-Cytokine Therapy for the Treatment of Asthma and Allergic Disease. Cytokine 28(4-5): 152-157.
- Chung KF (2006) Cytokines as Targets in Chronic Obstructive Pulmonary Disease. Current Drug Targets 7(6): 675-681.
- Wang Xiaowen, Xin Jie Chen (2015) A Cytosolic Network Suppressing Mitochondria-Mediated Proteostatic Stress and Cell Death. Nature 524 (7566): 481-484.
- Simões e Silva AC, KD Silveira, AJ Ferreira, MM Teixeira (2013) ACE2, Angiotensin-(1-7) and Mas Receptor Axis in Inflammation and Fibrosis. Br J Pharmacol 169 (3): 477-492.
- South Andrew M, Tammy M Brady, Joseph T Flynn (2020) ACE2, COVID-19, and ACE Inhibitor and ARB Use during the Pandemic. Hypertension 76(1): 16-22.
- Rodrigues Prestes, Thiago Ruiz, Natalia Pessoa Rocha, Aline Silva Miranda, Antônio Lúcio Teixeira, et al. (2017) The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Current Drug Targets 18 (11): 1301-1345.
- Delgado Roche Livan, Fernando Mesta (2020) Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Archives of Medical Research 51(5): 384-387.
- Imai Yumiko, Keiji Kuba, G Greg Neely, Rubina Yaghubian Malhami, Thomas Perkmann, et al. (2008) Identification of Oxidative Stress and Toll-like Receptor 4 Signaling as a Key Pathway of Acute Lung Injury. Cell 133(2): 235-249.
- Magro Cynthia, J Justin Mulvey, David Berlin, Gerard Nuovo, Steven Salvatore, et al. (2020) Complement Associated Microvascular Injury and Thrombosis in the Pathogenesis of Severe COVID-19 Infection: A Report of Five Cases. Translational Research: The Journal of Laboratory and Clinical Medicine 220: 1-13.
- Huppert Laura A, Michael A Matthay, Lorraine B Ware (2019) Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med 40 (1): 31-39.
- Windsor AC, PG Mullen, AA Fowler, HJ Sugerman (1993) Role of the Neutrophil in Adult Respiratory Distress Syndrome. The British Journal of Surgery 80 (1): 10-17.
- Gasparini Chiara, Marc Feldmann (2012) NF-κB as a Target for Modulating Inflammatory Responses. Current Pharmaceutical Design 18 (35): 5735-5745.
- Park Hee Sun, So Ri Kim, Yong Chul Lee (2009) Impact of Oxidative Stress on Lung Diseases. Respirology 14 (1): 27–38.
- Hulsmann AR, HR Raatgeep, JC den Hollander, T Stijnen, PR Saxena, et al. (1994) Oxidative Epithelial Damage Produces Hyperresponsiveness of Human Peripheral Airways. American Journal of Respiratory and Critical Care Medicine 149(2 Pt 1): 519-525.
- Cho Sungsam, Yoshishige Urata, Tetsuya Iida, Shinji Goto, Michiko Yamaguchi, et al. (1998) Glutathione Downregulates the Phosphorylation of IκB: Autoloop Regulation of the NF-κB-Mediated Expression of NF-κB Subunits by TNF-α in Mouse Vascular Endothelial Cells. Biochemical and Biophysical Research Communications. 253(1): 104-108.
- Rahman Arshad, Fabeha Fazal (2011) Blocking NF-κB: An Inflammatory Issue. Proceedings of the American Thoracic Society 8 (6): 497-503.
- Yu BP (1995) Cellular Defenses Against Damage from Reactive Oxygen Species. Physiological Reviews 75(1): 236.
- Ames BN, MK Shigenaga, TM Hagen (1993) Oxidants, Antioxidants, and the Degenerative Diseases of Aging. Proceedings of the National Academy of Sciences of the United States of America 90(17): 7915–7922.
- Zafarullah M, WQ Li, J Sylvester, M Ahmad (2003) Molecular Mechanisms of N-Acetylcysteine Actions. Cellular and Molecular Life Sciences: CMLS 60(1): 6-20.
- Davis W, Jr, Z Ronai, KD Tew (2001) Cellular Thiols and Reactive Oxygen Species in Drug-Induced Apoptosis. The Journal of Pharmacology and Experimental Therapeutics 296 (1): 1-6.
- Li WQ, F Dehnade, M Zafarullah (2000) Thiol Antioxidant, N-Acetylcysteine, Activates Extracellular Signal-Regulated Kinase Signaling Pathway in Articular Chondrocytes. Biochemical and Biophysical Research Communications 275(3): 789-794.
- Aruoma OI, B Halliwell, BM Hoey, J Butler (1989) The Antioxidant Action of N-Acetylcysteine: Its Reaction with Hydrogen Peroxide, Hydroxyl Radical, Superoxide, and Hypochlorous Acid. Free Radical Biology & Medicine 6(6): 593-597.
- Blackwell TS, TR Blackwell, EP Holden, BW Christman, JW Christman, et al. (1996) In Vivo Antioxidant Treatment Suppresses Nuclear Factor-Kappa B Activation and Neutrophilic Lung Inflammation. Journal of Immunology 157(4): 1630–1637.
- Hashimoto S, Y Gon, K Matsumoto, I Takeshita, T Machino, et al. (2001) Intracellular Glutathione Regulates Tumour Necrosis Factor-Alpha-Induced p38 MAP Kinase Activation and RANTES Production by Human Bronchial Epithelial Cells. Clinical Experimental Allergy.
- Lo YY, JM Wong, TF Cruz (1996) Reactive Oxygen Species Mediate Cytokine Activation of c-Jun NH2-Terminal Kinases. The Journal of Biological Chemistry 271(26): 15703–15707.
- Lo YY, JA Conquer, S Grinstein, TF Cruz (1998) Interleukin-1 Beta Induction of c-Fos and Collagenase Expression in Articular Chondrocytes: Involvement of Reactive Oxygen Species. Journal of Cellular Biochemistry 69(1): 19–29.
- Zhang Ying, Shaoxue Ding, Caifeng Li, Yifeng Wang, Zhe Chen, et al. (2017) Effects of N-Acetylcysteine Treatment in Acute Respiratory Distress Syndrome: A Meta-Analysis. Exp Ther Med 14 (4): 2863–2868.
- Auten Richard L, Jonathan M Davis (2009) Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatric Research 66(2): 121–127.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.